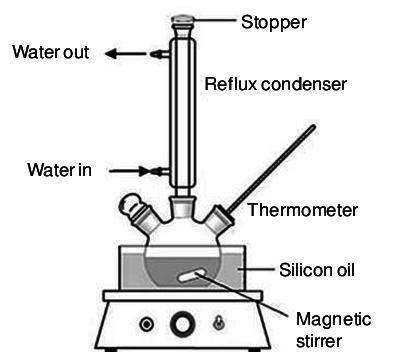
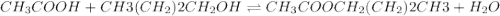Which one of the following choices describes most accurately the actual, internal reaction temperature (in other words, the temperature of the reaction mixture inside the reaction vial) for the Fischer esterification experiment of 1-butanol with acetic acid to form n-butyl acetate? Select one, and explain your answer.
a) Sand bath temperature (160-180 °C)
b) Boiling point of 1-butanol (116-118 °C)
c) Boiling point of the reaction mixture (reflux temperature)
d) Boiling point of acetic acid (117 °C)
e) Boiling point of n-butyl acetate (124-126 °C)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Which one of the following choices describes most accurately the actual, internal reaction temperatu...
Questions



Mathematics, 23.04.2020 21:26




Mathematics, 23.04.2020 21:26


History, 23.04.2020 21:26

Geography, 23.04.2020 21:26

Chemistry, 23.04.2020 21:26



Physics, 23.04.2020 21:26


History, 23.04.2020 21:26



Mathematics, 23.04.2020 21:26

Mathematics, 23.04.2020 21:26