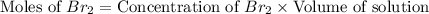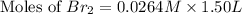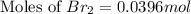Chemistry, 19.03.2020 01:16 blackwhiteroses383
Consider the reaction. 2 HBr(g) ¡ H2(g) + Br2(g) a. Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. In the first 25.0 s of this reaction, the concentration of HBr drops from 0.600 M to 0.512 M. Calculate the average rate of the reaction during this time interval. c. If the volume of the reaction vessel in part b is 1.50 L, what amount of Br2 (in moles) forms during the first 15.0 s of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
Consider the reaction. 2 HBr(g) ¡ H2(g) + Br2(g) a. Express the rate of the reaction in terms of the...
Questions

Mathematics, 06.03.2020 05:23







Biology, 06.03.2020 05:25



Mathematics, 06.03.2020 05:27

Geography, 06.03.2020 05:37

Biology, 06.03.2020 05:39







![Rate=-\frac{1}{2}\frac{d[HBr]}{dt}=+\frac{d[H_2]}{dt}=+\frac{d[Br_2]}{dt}](/tpl/images/0553/1566/27c4e.png)
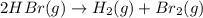
![\text{Rate of disappearance of }HBr=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0553/1566/d63dd.png)
![\text{Rate of disappearance of }H_2=+\frac{d[H_2]}{dt}](/tpl/images/0553/1566/eb73c.png)
![\text{Rate of formation of }Br_2=+\frac{d[Br_2]}{dt}](/tpl/images/0553/1566/30b3c.png)
![\text{Average rate}=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0553/1566/79555.png)
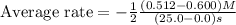
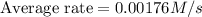
![\frac{d[Br_2]}{dt}=0.00176M/s](/tpl/images/0553/1566/78ef0.png)
![\frac{d[Br_2]}{15.0s}=0.00176M/s](/tpl/images/0553/1566/22daf.png)
![[Br_2]=0.00176M/s\times 15.0s](/tpl/images/0553/1566/5d9c4.png)
![[Br_2]=0.0264M](/tpl/images/0553/1566/42226.png)