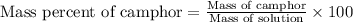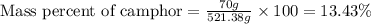Chemistry, 19.03.2020 01:36 briseisr20
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the camphor tree. Assume you dissolve 70.0 g of camphor (C10H16O) in 575 mL of ethanol, C2H5OH. Calculate the molarity, molality, mole fraction, and weight percentage of camphor in this solution. (The density of ethanol is 0.785 g/mL.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the...
Questions

Mathematics, 10.12.2021 23:30


Biology, 10.12.2021 23:30


English, 10.12.2021 23:30

Mathematics, 10.12.2021 23:30

English, 10.12.2021 23:30


English, 10.12.2021 23:30

Spanish, 10.12.2021 23:30

History, 10.12.2021 23:30

Computers and Technology, 10.12.2021 23:30


Computers and Technology, 10.12.2021 23:30


English, 10.12.2021 23:30

Chemistry, 10.12.2021 23:30



Mathematics, 10.12.2021 23:30

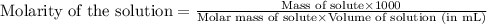
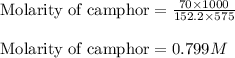
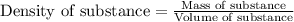
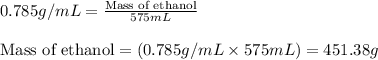
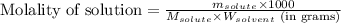
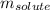 = Given mass of solute (camphor) = 70 g
= Given mass of solute (camphor) = 70 g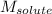 = Molar mass of solute (camphor) = 152.2 g/mol
= Molar mass of solute (camphor) = 152.2 g/mol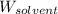 = Mass of solvent (ethanol) = 451.38 g
= Mass of solvent (ethanol) = 451.38 g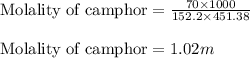
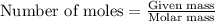 .....(1)
.....(1)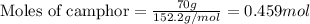
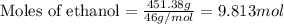
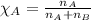
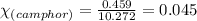 \
\