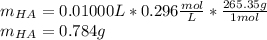Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Thiamine hydrochloride (C12H18ON4SCl2) is a water-soluble form of thiamine (vitamin B1; Ka = 3.37×10...
Questions

Mathematics, 30.01.2021 07:10

Biology, 30.01.2021 07:10

Mathematics, 30.01.2021 07:10

History, 30.01.2021 07:10


English, 30.01.2021 07:10

Mathematics, 30.01.2021 07:10


Mathematics, 30.01.2021 07:10

Mathematics, 30.01.2021 07:10

English, 30.01.2021 07:10

Biology, 30.01.2021 07:10

Mathematics, 30.01.2021 07:10

Mathematics, 30.01.2021 07:10



Mathematics, 30.01.2021 07:10


Social Studies, 30.01.2021 07:10

English, 30.01.2021 07:10

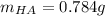
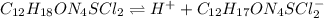
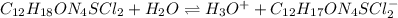
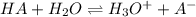
![pH=pKa+log(\frac{[A^-]}{[HA]} )](/tpl/images/0553/3571/4a01a.png)
![log(\frac{[A^-]}{[HA]} )=3.50-[-log(3.37x 10^{-7})]=3.50-6.47=-2.97}\\\\\frac{[A^-]}{[HA]} =10^{-2.97}=1.07x10^{-3}](/tpl/images/0553/3571/b4cdb.png)
![[A^-]=1.07x10^{-3}}[HA]](/tpl/images/0553/3571/1acb8.png)
![[H]^+=[A^-]=10^{-pH}=10^{-3.50}=3.16x10^{-4}M](/tpl/images/0553/3571/d3a0e.png)
![[HA]=\frac{3.16x10^{-4}M}{1.07x10^{-3}} =0.296M](/tpl/images/0553/3571/7981c.png)