
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Which of the following statements is/are true? Adding AgNO3 to the solution will cause more Ag2S to dissolve. Adding some more Ag2S to the solution will cause more Ag2S to dissolve. Adding some water to the solution will cause more Ag2S to dissolve.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Wh...
Questions



Mathematics, 19.09.2019 18:30


Mathematics, 19.09.2019 18:30

English, 19.09.2019 18:30


History, 19.09.2019 18:30



English, 19.09.2019 18:30


Biology, 19.09.2019 18:30


Arts, 19.09.2019 18:30

Physics, 19.09.2019 18:30


Mathematics, 19.09.2019 18:30

History, 19.09.2019 18:30

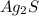 to dissolve is a true statement.
to dissolve is a true statement.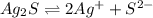
![K_{sp}=[Ag^{+}]^{2}[S^{2-}]](/tpl/images/0553/3669/0192a.png)
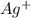 increases when
increases when 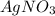 is added into solution. But value of
is added into solution. But value of 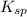 is constant at a certain temperature. Hence to keep
is constant at a certain temperature. Hence to keep 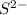 to produce more
to produce more 

