Chemistry, 19.03.2020 02:51 Itsyourgirllulu
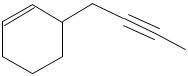
An optically active alkyne A (C10H14) can be catalytically hydrogenated to butylcyclohexane. Treatment of A with C2H5MgBr liberates no gas. Catalytic hydrogenation of A over Pd/C in the presence of quinoline poison and treatment of the product B with O3 and then H2O2 gives an optically active tricarboxylic acid C8H12O6. (A tricarboxylic acid is a compound with three –CO2H groups.) Give the structure of A (without stereochemistry).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
You know the right answer?
An optically active alkyne A (C10H14) can be catalytically hydrogenated to butylcyclohexane. Treatme...
Questions

Mathematics, 25.01.2020 06:31




Mathematics, 25.01.2020 06:31

Mathematics, 25.01.2020 06:31

Chemistry, 25.01.2020 06:31








Mathematics, 25.01.2020 06:31


Social Studies, 25.01.2020 06:31


Chemistry, 25.01.2020 06:31