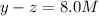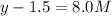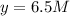Chemistry, 19.03.2020 03:07 DVM117x017
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain temperature by the reaction
3H2(g) +N2(g) <=> 2NH3(g)
At equilibrium the concentrations are [H2]=5.0 M, [N2]= 8.0M, [NH3]=3.0M
What were the concentrations of nitrogen gas and hydrogen gas that were reacted initially.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain tem...
Questions

Mathematics, 19.08.2019 05:00


History, 19.08.2019 05:00


Mathematics, 19.08.2019 05:00




History, 19.08.2019 05:00

History, 19.08.2019 05:00

Mathematics, 19.08.2019 05:00


Mathematics, 19.08.2019 05:00


Physics, 19.08.2019 05:00

Mathematics, 19.08.2019 05:00

Health, 19.08.2019 05:00

English, 19.08.2019 05:00

Social Studies, 19.08.2019 05:00

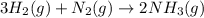
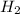 =
= 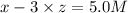
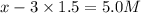
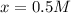
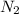 =
=