
Chemistry, 19.03.2020 04:08 maskythegamer
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in the atmosphere occur mainly in regions with large automobile or power plant emissions. The equilibrium constant for the reaction of N2 and 02 to give NO is very small. The reaction is, however, highly endothermic, with a heat of reaction equal to +180 kJ (Equation 7). N2(g) + O2(g) 180 kJ 2NO(g) Equation 7 +
(a) Use LeChâtelier's Principle to explain why the concentration of NO at equilibrium increases when the reaction takes place at high temperatures.
(b) Use LeChâtelier's Principle to predict whether the concentration of NO at equilibrium should increase when the reaction takes place at high pressures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in th...
Questions

Mathematics, 13.07.2019 15:30


Mathematics, 13.07.2019 15:30


Mathematics, 13.07.2019 15:30


Biology, 13.07.2019 15:30


Mathematics, 13.07.2019 15:30


Mathematics, 13.07.2019 15:30


Physics, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30

Spanish, 13.07.2019 15:30



Mathematics, 13.07.2019 15:30


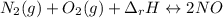
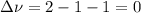
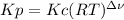
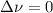 ,
, 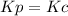 , so no effect in concentration is due to the pressure.
, so no effect in concentration is due to the pressure.


