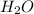The following reaction has Kc > 1: Ni(H2O)62+(aq) + 6 NH3(aq) → Ni(NH3)62+(aq) + 6 H2O(l) Based on this information, what can you conclude about the strength of NH3 as a Lewis acid/base? Select all that apply. Group of answer choices NH3 is a stronger Lewis base than H2O NH3 is a weaker Lewis base than H2O NH3 is a stronger Lewis acid than H2O NH3 is a weaker Lewis acid than H2O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
The following reaction has Kc > 1: Ni(H2O)62+(aq) + 6 NH3(aq) → Ni(NH3)62+(aq) + 6 H2O(l) Based o...
Questions



History, 29.10.2020 23:10


History, 29.10.2020 23:10


Mathematics, 29.10.2020 23:10


Mathematics, 29.10.2020 23:10





Law, 29.10.2020 23:20




Mathematics, 29.10.2020 23:20

History, 29.10.2020 23:20

History, 29.10.2020 23:20

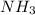 is a stronger Lewis base than
is a stronger Lewis base than