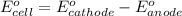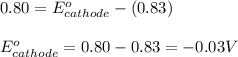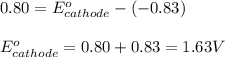Chemistry, 19.03.2020 03:59 hardwick744
A certain half-reaction has a standard reduction potential E⁰ʀᴇᴅ = 0.83 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that must provide at least 0.80V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell.
(1) Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".
(2) Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
A certain half-reaction has a standard reduction potential E⁰ʀᴇᴅ = 0.83 V. An engineer proposes usin...
Questions


Mathematics, 22.10.2019 22:10


Mathematics, 22.10.2019 22:10

Mathematics, 22.10.2019 22:10

Arts, 22.10.2019 22:10



History, 22.10.2019 22:10



Mathematics, 22.10.2019 22:10



History, 22.10.2019 22:10

Mathematics, 22.10.2019 22:10


Biology, 22.10.2019 22:10

English, 22.10.2019 22:10

Mathematics, 22.10.2019 22:10

 of the reaction, we use the equation:
of the reaction, we use the equation: