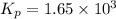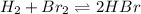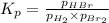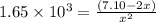Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
The equilibrium constant for the following reaction: H2(g) + Br2(g) ↔ 2HBr (g) is 1.65 x 103 at a ce...
Questions

Mathematics, 05.10.2020 15:01

Social Studies, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01


Biology, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01





Mathematics, 05.10.2020 15:01

Physics, 05.10.2020 15:01

Physics, 05.10.2020 15:01

History, 05.10.2020 15:01

History, 05.10.2020 15:01


Biology, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01