
Chemistry, 19.03.2020 03:48 kaywendel2008
For the reaction hconh2(g)⇌nh3(g)+co(g), k= 4.84 at 400 k what can be said about this reaction at this temperature? view available hint(s) for the reaction , at 400 what can be said about this reaction at this temperature? the equilibrium lies far to the right. the reaction will proceed very slowly. the reaction contains significant amounts of products and reactants at equilibrium. the equilibrium lies far to the left.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
For the reaction hconh2(g)⇌nh3(g)+co(g), k= 4.84 at 400 k what can be said about this reaction at th...
Questions





Chemistry, 13.03.2021 01:00

English, 13.03.2021 01:00




Mathematics, 13.03.2021 01:00



Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00




English, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

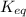
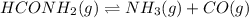
![K_{eq}=\frac{[NH_3]\times [CO]}{[HCONH_2]}](/tpl/images/0553/4863/2c193.png)
![4.84=\frac{[NH_3]\times [CO]}{[HCONH_2]}](/tpl/images/0553/4863/48469.png)


