Chemistry, 19.03.2020 04:59 eduardo2433
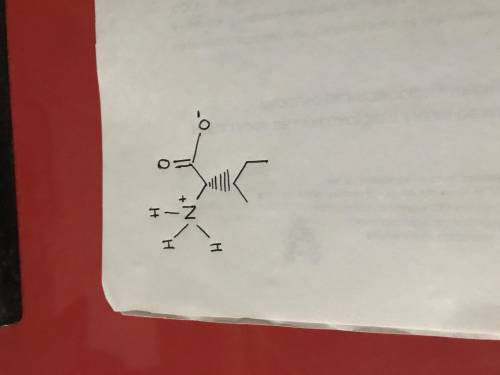
The amino acid isoleucine has pKa values of 2.32 (–COOH) and 9.76 (–NH3+). What is the predominant form of the amino acid at pH = 6.0? Fill in any missing hydrogen atoms and/or charges on the provided structure to adjust the structure to reflect a pH of 6.0.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
The amino acid isoleucine has pKa values of 2.32 (–COOH) and 9.76 (–NH3+). What is the predominant f...
Questions


Mathematics, 17.01.2020 19:31

Geography, 17.01.2020 19:31


Mathematics, 17.01.2020 19:31


Mathematics, 17.01.2020 19:31




Mathematics, 17.01.2020 19:31


English, 17.01.2020 19:31

Mathematics, 17.01.2020 19:31

English, 17.01.2020 19:31

Social Studies, 17.01.2020 19:31

History, 17.01.2020 19:31