
Chemistry, 19.03.2020 06:31 nikejenkins18
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compartment and 0.600 mole of n2(g) at 100.08c and 2.00 atm in the other compartment. these compartments are connected by a partition that transmits heat. what is the final tempera-ture in the box at thermal equilibrium? [for he(g), cv 5 12.5 j k 21mol 21 ; for n2(g), cv 5 20.7 j k 21mol 21

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

You know the right answer?
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compart...
Questions




English, 23.03.2020 23:09







Mathematics, 23.03.2020 23:09

Mathematics, 23.03.2020 23:09

Mathematics, 23.03.2020 23:09

Mathematics, 23.03.2020 23:09






English, 23.03.2020 23:09

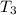 = 341.08 K
= 341.08 K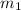 = 0.4 mole
= 0.4 mole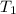 = 20.08 °c = 293.08 K
= 20.08 °c = 293.08 K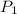 = 1 atm = 101.325 K pa
= 1 atm = 101.325 K pa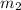 = 0.6 mole
= 0.6 mole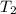 = 373.08 K
= 373.08 K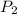 = 2 atm = 202 K pa
= 2 atm = 202 K pa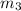 =
= 

