
Chemistry, 19.03.2020 06:48 JohnStewart714
Compute the equilibrium constant, K, at 25 ∘C for the reaction between Ni2+(aq) and Zn(s), which form Ni(s) and Zn2+(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

You know the right answer?
Compute the equilibrium constant, K, at 25 ∘C for the reaction between Ni2+(aq) and Zn(s), which for...
Questions

Physics, 25.01.2020 21:31

History, 25.01.2020 21:31




Mathematics, 25.01.2020 21:31


Mathematics, 25.01.2020 21:31


English, 25.01.2020 21:31

Mathematics, 25.01.2020 21:31

Mathematics, 25.01.2020 21:31

Business, 25.01.2020 21:31


Physics, 25.01.2020 21:31


Social Studies, 25.01.2020 21:31

Mathematics, 25.01.2020 21:31

Mathematics, 25.01.2020 21:31

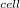 =
= 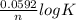
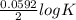
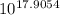 = 2.04×10¹⁷.
= 2.04×10¹⁷. 

