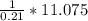A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate the molar composition of this gas on both awet and a dry basis and the ratio (mol H2O/ mol dry gas). (b) If100kg/30%excessair,(kmol/ h)? How would the answer change if the combustion were only 75% complete?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate t...
Questions








Mathematics, 10.07.2019 09:30





Mathematics, 10.07.2019 09:30


Physics, 10.07.2019 09:30



Biology, 10.07.2019 09:30

Chemistry, 10.07.2019 09:30

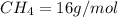
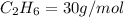
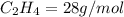
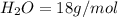
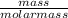
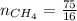
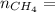 4.69 moles
4.69 moles 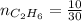
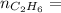 0.33 moles
0.33 moles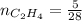
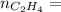 0.18 moles
0.18 moles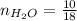
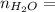 0.56 moles
0.56 moles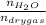
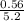




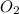 required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles
required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles