
Chemistry, 19.03.2020 07:42 kcceff4085
A food product is being frozen in a system capable of removing 6000 kJ of thermal energy. The product has a specifi c heat of 4 kJ/(kg C) above the freezing temperature of 2 C, the latent heat of fusion equals 275 kJ/kg, and the frozen product has a specifi c heat of 2.5 kJ/(kg C) below 2 C. If 10 kg of product enters the system at 10C, determine the exit temperature of the product..

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1


Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
A food product is being frozen in a system capable of removing 6000 kJ of thermal energy. The produc...
Questions




Mathematics, 29.05.2021 04:40


World Languages, 29.05.2021 04:40


Biology, 29.05.2021 04:40

Biology, 29.05.2021 04:40





History, 29.05.2021 04:40


Mathematics, 29.05.2021 04:40


English, 29.05.2021 04:40


English, 29.05.2021 04:40

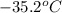

 = specific heat of liquid =
= specific heat of liquid = 
 = latent heat of fusion =
= latent heat of fusion = 
 = specific heat of frozen =
= specific heat of frozen = 
 = initial temperature of liquid =
= initial temperature of liquid = 
 = final temperature of liquid =
= final temperature of liquid = 
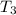 = initial temperature of frozen = ?
= initial temperature of frozen = ? = final temperature of frozen =
= final temperature of frozen = ![6000kJ=[15kg\times 4kJ/kg^oC\times (10-2)^oC]+[15kg\times 275kJ/kg]+[15kg\times 2.5kJ/kg^oC\times (2-T_3)^oC]](/tpl/images/0553/7186/e8cbe.png)



