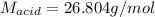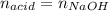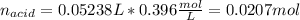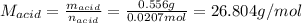Chemistry, 19.03.2020 07:52 quickestlearner6171
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 52.38 mL of 0.396 M NaOH. Calculate the molar mass of the acid, assuming it to be a monoprotic acid

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

You know the right answer?
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 5...
Questions


Mathematics, 24.12.2021 04:10

Biology, 24.12.2021 04:10






History, 24.12.2021 04:10

Chemistry, 24.12.2021 04:10

SAT, 24.12.2021 04:10

SAT, 24.12.2021 04:20



Social Studies, 24.12.2021 04:20


Physics, 24.12.2021 04:20