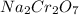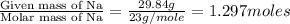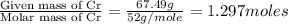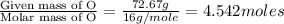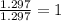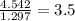Chemistry, 19.03.2020 08:29 AdanNava699
A 170.00g sample of an unidentified compound contains 29.84g sodium,67.49g chromium, and 72.67g oxygen. find the emperical formula.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
A 170.00g sample of an unidentified compound contains 29.84g sodium,67.49g chromium, and 72.67g oxyg...
Questions


English, 03.01.2022 03:10

Chemistry, 03.01.2022 03:10

Mathematics, 03.01.2022 03:10

Mathematics, 03.01.2022 03:10



Biology, 03.01.2022 03:10

Mathematics, 03.01.2022 03:10


Mathematics, 03.01.2022 03:20


Mathematics, 03.01.2022 03:20

Mathematics, 03.01.2022 03:20

Mathematics, 03.01.2022 03:20



Mathematics, 03.01.2022 03:20


Mathematics, 03.01.2022 03:20