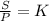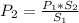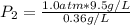2. A gas has a solubility in water
at 0°C of 3.6 g/L at a pressure
of 1.0 atm. What pressu...

Chemistry, 19.03.2020 09:05 queenkimm26
2. A gas has a solubility in water
at 0°C of 3.6 g/L at a pressure
of 1.0 atm. What pressure is
needed to produce an aqueous
solution containing 9.5 g/L of
the same gas at 0°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Questions


English, 21.05.2020 04:01

Mathematics, 21.05.2020 04:01



Mathematics, 21.05.2020 04:01

Mathematics, 21.05.2020 04:01

Mathematics, 21.05.2020 04:01


Computers and Technology, 21.05.2020 04:01


Mathematics, 21.05.2020 04:01



Mathematics, 21.05.2020 04:01


Chemistry, 21.05.2020 04:01

Mathematics, 21.05.2020 04:01