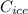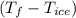2. A 10.0 g ice cube, initially at 0.0ºC, is melted in 100.0 g of water that was initially 20.0ºC. After the ice has melted, the equilibrium temperature is 10.93ºC. Show your work!
a. Calculate the total heat (in J) lost by the water (specific heat for water is 4.184 J/g°C).
b. Calculate the heat gained by the ice cube after it melts (specific heat for ice is 2.093 J/g°C).
c. Calculate the heat it took to melt the ice. (It takes 334.0 J/g of heat energy to melt ice). qmelt = mice ΔHmelt

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
2. A 10.0 g ice cube, initially at 0.0ºC, is melted in 100.0 g of water that was initially 20.0ºC. A...
Questions



Mathematics, 06.03.2020 12:45


English, 06.03.2020 12:45

Mathematics, 06.03.2020 12:46

Geography, 06.03.2020 12:46


Medicine, 06.03.2020 12:46



Mathematics, 06.03.2020 12:47

English, 06.03.2020 12:47


Mathematics, 06.03.2020 12:47


History, 06.03.2020 12:48

Mathematics, 06.03.2020 12:48


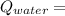 3.8 KJ
3.8 KJ 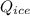 = 228.76 J
= 228.76 J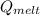 = 3340 J
= 3340 J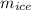 = 10 gm
= 10 gm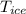 = 0 °c
= 0 °c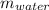 = 100 gm
= 100 gm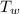 = 20 °c
= 20 °c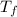 = 10.93 °c
= 10.93 °c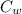 (
(