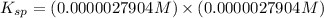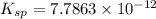Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
. Calculate Ksp for copper oxalate (CuC2O4). Use your calculated copper ion concentration (0.0000027...
Questions



Mathematics, 25.07.2021 20:30

Physics, 25.07.2021 20:30

Social Studies, 25.07.2021 20:30

History, 25.07.2021 20:30


Biology, 25.07.2021 20:30


Arts, 25.07.2021 20:30

Mathematics, 25.07.2021 20:30

French, 25.07.2021 20:30


Mathematics, 25.07.2021 20:30

Social Studies, 25.07.2021 20:30


Mathematics, 25.07.2021 20:30

Mathematics, 25.07.2021 20:30


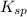 is
is 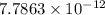
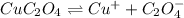
![K_{sp}=[Cu^{+}][C_2O_4^{-}]](/tpl/images/0553/9563/c6650.png)