

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

You know the right answer?
1. 51.2 g of NaClO (pKa of HClO is 7.50) and 25.3 g of KF (pKa of of HF is 3.17) were combined in en...
Questions




History, 19.07.2019 11:20


English, 19.07.2019 11:20

Chemistry, 19.07.2019 11:20



History, 19.07.2019 11:20



Mathematics, 19.07.2019 11:20

Mathematics, 19.07.2019 11:20


English, 19.07.2019 11:20

Social Studies, 19.07.2019 11:20

Mathematics, 19.07.2019 11:20

Biology, 19.07.2019 11:20

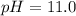
![[HClO]_{eq}=9.32x10^{-4}M](/tpl/images/0553/9989/0839d.png)
![[HF]_{eq}=5.07x10^{-6}M](/tpl/images/0553/9989/a410e.png)

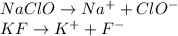
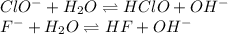

![Kb_{NaClO}=3.16x10^{-7}=\frac{[HClO][OH]^-}{[ClO^-]}=\frac{(x_{ClO})(x_{ClO})}{2.75-x_{ClO}}\\Kb_{KF}=1.48x10^{-11}=\frac{[HF][OH]^-}{[F^-]}=\frac{(x_{F})(x_{F})}{1.74-x_{F}}](/tpl/images/0553/9989/f20e7.png)
 for both the ClO⁻ and F⁻ equilibriums, we obtain:
for both the ClO⁻ and F⁻ equilibriums, we obtain:
![[OH^-]_{tot}=9.32x10^{-4}M+5.07x10^{-6}M=9.37x10^{-4}M](/tpl/images/0553/9989/37b16.png)
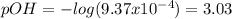

![[HClO]_{eq}=x_{ClO}=9.32x10^{-4}M](/tpl/images/0553/9989/36a6b.png)
![[HF]_{eq}=x_{F}=5.07x10^{-6}M](/tpl/images/0553/9989/7dd4a.png)


