Chemistry, 19.03.2020 09:31 priscillarios30
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g) Calculate the equilibrium concentrations of reactant and products when 0.372 moles of HI are introduced into a 1.00 L vessel at 698 K. [HI] = M [H2] = M [I2] = M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g)...
Questions


Mathematics, 28.01.2021 23:40

Social Studies, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40


Engineering, 28.01.2021 23:40

History, 28.01.2021 23:40

Physics, 28.01.2021 23:40

SAT, 28.01.2021 23:40

History, 28.01.2021 23:40


Mathematics, 28.01.2021 23:40




Mathematics, 28.01.2021 23:40



Mathematics, 28.01.2021 23:40

History, 28.01.2021 23:40

![[HI]=(0.372-2x) M =(0.372-2\times 0.03935)M =0.2933 M](/tpl/images/0553/9717/216fb.png)
![[H_2]=x = 0.03935 M](/tpl/images/0553/9717/0d5d5.png)
![[I_2]=x = 0.03935 M](/tpl/images/0553/9717/d2ad6.png)
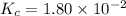
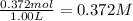
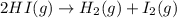
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0553/9717/ef85e.png)