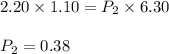Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
3. 2.20 L of air at 1.10 atm is allowed to expand to fill a 6.30 L container. Find the final pressur...
Questions

Mathematics, 28.01.2021 23:30





Mathematics, 28.01.2021 23:30





History, 28.01.2021 23:30

English, 28.01.2021 23:30



Computers and Technology, 28.01.2021 23:30


English, 28.01.2021 23:30

Mathematics, 28.01.2021 23:30

Mathematics, 28.01.2021 23:30