

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
How many total electrons are shared between two nitrogen atoms when they bond to form a diatomic nit...
Questions






Mathematics, 12.04.2020 19:45


Mathematics, 12.04.2020 19:45






Mathematics, 12.04.2020 19:46


History, 12.04.2020 19:46


Mathematics, 12.04.2020 19:46


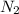 .
.

