
Chemistry, 19.03.2020 17:10 studybuddy0203
Predict the effect of increasing the container volume on the amounts of each reactant and product in the following reactions: (a) CH3OH(l) <--> CH3OH(g) (b) CH4(g) + NH3(g) <--> HCN(g) + 3H2(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

You know the right answer?
Predict the effect of increasing the container volume on the amounts of each reactant and product in...
Questions



Social Studies, 08.04.2020 16:02

English, 08.04.2020 16:02


Mathematics, 08.04.2020 16:02


Mathematics, 08.04.2020 16:02


Mathematics, 08.04.2020 16:04



Biology, 08.04.2020 16:13

Mathematics, 08.04.2020 16:14

Mathematics, 08.04.2020 16:14


English, 08.04.2020 16:14

Mathematics, 08.04.2020 16:14




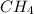 and 1 mol of
and 1 mol of 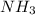 ) and in the right side we will have 4 moles (1 mol of
) and in the right side we will have 4 moles (1 mol of  and 3 mol of
and 3 mol of 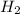 ). Therefore, the reaction will move to the right side.
). Therefore, the reaction will move to the right side.


