Chemistry, 19.03.2020 17:08 Levantine3667
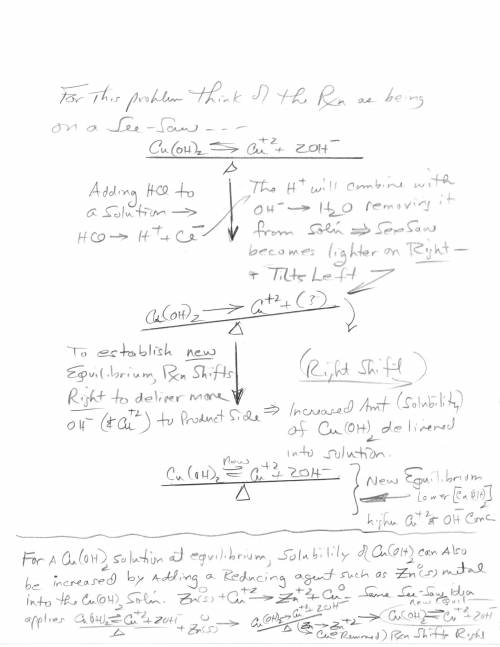
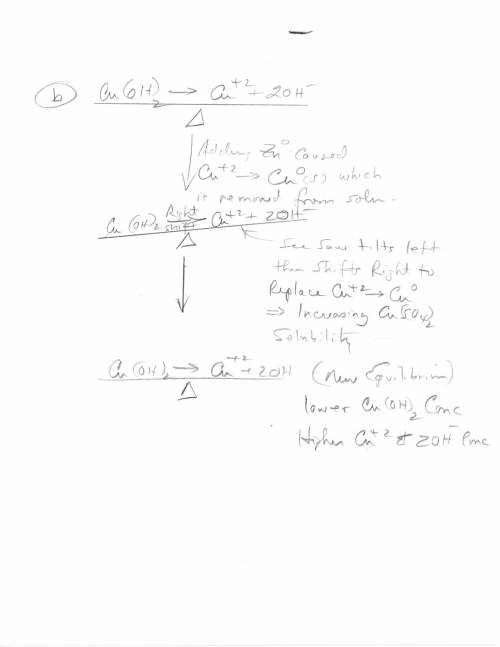
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly soluble. Cu(OH)2(s) Cu2 (aq) 2OH- (aq) a. Explain how the solubility can be increased by adding HCl to the solution. b. Explain how the concentration of copper (II) ion or of hydroxide ion can be reduced in the solution so that more of the solid copper hydroxide can be dissolved.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2


You know the right answer?
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly...
Questions

Computers and Technology, 22.01.2021 05:20

English, 22.01.2021 05:20


Mathematics, 22.01.2021 05:20

Mathematics, 22.01.2021 05:20

Mathematics, 22.01.2021 05:20

Mathematics, 22.01.2021 05:20



History, 22.01.2021 05:20


Biology, 22.01.2021 05:20

Advanced Placement (AP), 22.01.2021 05:20

Chemistry, 22.01.2021 05:20

Social Studies, 22.01.2021 05:20

Arts, 22.01.2021 05:20


Mathematics, 22.01.2021 05:20

Mathematics, 22.01.2021 05:20