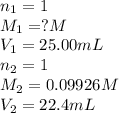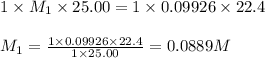Chemistry, 19.03.2020 18:34 wafflewarriormg
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and the equivalence point volume was determined by graphical means to be 22.4 mL. What is the concentration of the acetic acid?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2
You know the right answer?
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and...
Questions




Social Studies, 08.07.2021 15:30




History, 08.07.2021 15:40




Mathematics, 08.07.2021 15:40







Computers and Technology, 08.07.2021 15:40

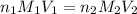
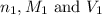 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 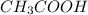
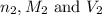 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.