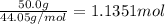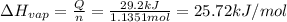Chemistry, 19.03.2020 20:37 rexerlkman4145
29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas phase. The molecular
weight of acetaldehyde is 44.05 g/mol. What is the heat of
vaporization of acetaldehyde in kJ/mol?
kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas...
acetaldehyde from the liquid to gas...
Questions

Mathematics, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50



Biology, 01.03.2021 21:50



Mathematics, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50


Mathematics, 01.03.2021 21:50


Social Studies, 01.03.2021 21:50


Business, 01.03.2021 21:50



Spanish, 01.03.2021 21:50