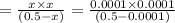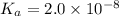Chemistry, 19.03.2020 20:40 afosburgh20
50 POINTS PLEASE HELP ASAP! I WILL MARK BRAINLIEST ONLY IF CORRECT AND CLEARLY STATED
19. Calculate the hydrogen-ion concentration [H^+] for the aqueous solution in which [OH^-] is 1 x 10^-11 mol/L. Is this solution acidic, basic, or neutral? Show your work. (3 points)
20. Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5 M solution of this acid gives a hydrogen-ion concentration of 0.000 1.M? Show your work. (3 points)
DO NOT Copy and past the work from another answer UNLESS you can clearly state all the steps. I've seen them all so I know If you copy.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
50 POINTS PLEASE HELP ASAP! I WILL MARK BRAINLIEST ONLY IF CORRECT AND CLEARLY STATED
1...
1...
Questions

Health, 20.01.2022 19:40



English, 20.01.2022 19:50



Mathematics, 20.01.2022 19:50

Mathematics, 20.01.2022 19:50


Mathematics, 20.01.2022 19:50

SAT, 20.01.2022 19:50

English, 20.01.2022 19:50

English, 20.01.2022 19:50




Computers and Technology, 20.01.2022 19:50

Physics, 20.01.2022 19:50


 .
.![[OH^-]=1\times 10^{-11}](/tpl/images/0554/4732/291a3.png)
![pOH=-\log[OH^-]](/tpl/images/0554/4732/fe336.png)
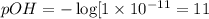
![pH=-\log[H^+]](/tpl/images/0554/4732/cf945.png)
![3=-\log[H^+]](/tpl/images/0554/4732/66d3f.png)
![[H^+]=10^{-3} M=0.001 M](/tpl/images/0554/4732/fcc5c.png)
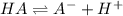
![[H^+]=x=0.0001 M](/tpl/images/0554/4732/b3468.png)
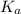 is given as:
is given as:![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0554/4732/a5cb9.png)