
Chemistry, 19.03.2020 20:33 tfaulk2884
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 732torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is 26.74 torr.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) I...
Questions

English, 18.09.2020 05:01

History, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

History, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

English, 18.09.2020 05:01

Biology, 18.09.2020 05:01

Social Studies, 18.09.2020 05:01

English, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

English, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

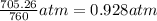
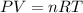
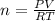
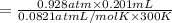
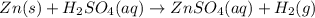
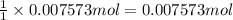 of zinc
of zinc

