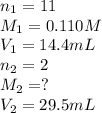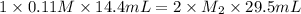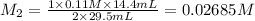Chemistry, 19.03.2020 20:29 adhitrfbvg
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydrochloric acid. If 29.5 mL of base are required to neutralize 14.4 mL of the acid, what is the molarity of the barium hydroxide solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydr...
Questions

Biology, 27.03.2021 01:50




Mathematics, 27.03.2021 01:50



Mathematics, 27.03.2021 01:50


Mathematics, 27.03.2021 01:50


Mathematics, 27.03.2021 01:50




Spanish, 27.03.2021 02:00

Mathematics, 27.03.2021 02:00



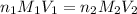
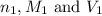 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 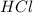
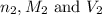 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 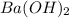 mm.
mm.