What is the [H3O+] in a solution with [OH-] = 1.0 x 10-12 M?
1.0 x 10-2 M
1....


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Questions

Mathematics, 26.03.2021 07:30


Biology, 26.03.2021 07:30




Mathematics, 26.03.2021 07:30


Mathematics, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30


History, 26.03.2021 07:30


Arts, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30

History, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30

Mathematics, 26.03.2021 07:30

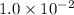 M.
M.![[OH^-]=1.0\times 10^{-12} M](/tpl/images/0554/5789/c2935.png)
![pOH=-\log[OH^-]](/tpl/images/0554/5789/fe336.png)
![pOH=-\log[1.0\times 10^{-12} M}]](/tpl/images/0554/5789/0b3e8.png)
![pH=-\log[H_3O^+]](/tpl/images/0554/5789/a23b5.png)
![2=-\log[H_3O^+]](/tpl/images/0554/5789/60ce9.png)
![[H_3O^+]=1.0\times 10^{-2} M](/tpl/images/0554/5789/2a366.png)


