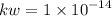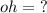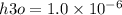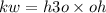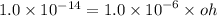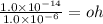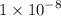1.0 x 10-6 M


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1


Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
What is the [OH-] in a solution that has a [H3O+] = 1.0 x 10-6 M?
1.0 x 10-6 M
1.0 x 10-6 M
Questions

World Languages, 26.12.2021 18:00

Social Studies, 26.12.2021 18:00


English, 26.12.2021 18:10


English, 26.12.2021 18:10

Mathematics, 26.12.2021 18:10

World Languages, 26.12.2021 18:10

English, 26.12.2021 18:10

Mathematics, 26.12.2021 18:10


English, 26.12.2021 18:10


English, 26.12.2021 18:20

Chemistry, 26.12.2021 18:20


Computers and Technology, 26.12.2021 18:20