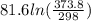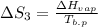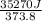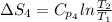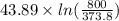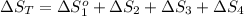The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-mol. Methanol boils at 337K with an enthalpy of vaporization of 35.270 kJ/mol at that temperature. The heat capacity of the vapor is 43.9 J/K-mol.__Calculate the entropy of one mole of methanol vapor at 800 K.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 12:50
What is the relative mass of an electron? a) 1/1840 the mass of a neutron + proton b) 1/1840 the mass of an alpha particle c) 1/1840 the mass of a c-12 atom d) 1/1840 the mass of a hydrogen atom
Answers: 3
You know the right answer?
The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-m...
Questions

Geography, 03.10.2019 04:10

Biology, 03.10.2019 04:10


French, 03.10.2019 04:10

Physics, 03.10.2019 04:10


Mathematics, 03.10.2019 04:10


Mathematics, 03.10.2019 04:10


Mathematics, 03.10.2019 04:10

Spanish, 03.10.2019 04:10



History, 03.10.2019 04:10





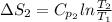 J/K mol
J/K mol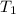 = 298 K,
= 298 K, 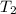 = 373.8 K
= 373.8 K