Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Consider the reaction for the combustion of benzene: 2 C6H6(g) 15 O2(g) ---> 12 CO2(g) 6 H2O(l) W...
Questions


Mathematics, 18.02.2020 17:13











Mathematics, 18.02.2020 17:13


Computers and Technology, 18.02.2020 17:13

Biology, 18.02.2020 17:13

Mathematics, 18.02.2020 17:13



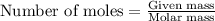 .....(1)
.....(1)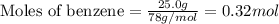
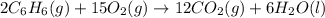
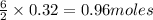 of water
of water