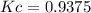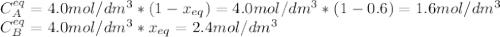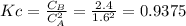The elementary reversible reaction:
2A <> B
is carried out isotherma...

The elementary reversible reaction:
2A <> B
is carried out isothermally and isobarically in a flow reactor where pure A is fed at a concentration of 4.0 mol/dm3. If the equilibrium conversion is found to be 60%. What is the equilibrium constant, Kc if the reaction is a gas phase reaction?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Questions


Mathematics, 15.12.2020 03:00



Social Studies, 15.12.2020 03:00

English, 15.12.2020 03:00


Geography, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00




Mathematics, 15.12.2020 03:00


Mathematics, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00

Arts, 15.12.2020 03:00

Social Studies, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00