
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions

Chemistry, 08.12.2020 01:00




English, 08.12.2020 01:00



Mathematics, 08.12.2020 01:00

History, 08.12.2020 01:00

History, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00

History, 08.12.2020 01:00


Social Studies, 08.12.2020 01:00






History, 08.12.2020 01:00

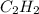 gas is often used in welding torches because of the very high heat produced when it reacts with oxygen
gas is often used in welding torches because of the very high heat produced when it reacts with oxygen 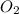 gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 1.5 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 1.5 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.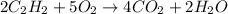
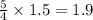 moles of oxygen
moles of oxygen

