
Chemistry, 19.03.2020 21:43 crebflower
Using only the following elements P, Br, and Mg, give the formulas for:A. an ionic compound. B. a molecular compound with polar covalent bonds that obeys the octet rule and has no formal charges.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Using only the following elements P, Br, and Mg, give the formulas for:A. an ionic compound. B. a mo...
Questions


Medicine, 02.03.2021 01:30

Arts, 02.03.2021 01:30


Geography, 02.03.2021 01:30




Health, 02.03.2021 01:30



Mathematics, 02.03.2021 01:30

Mathematics, 02.03.2021 01:30


Mathematics, 02.03.2021 01:30

Mathematics, 02.03.2021 01:30


Social Studies, 02.03.2021 01:30

Mathematics, 02.03.2021 01:30

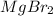 2. Polar molecular compound-
2. Polar molecular compound- 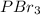
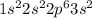 . The outer most shell of this element has 2 electrons so it loses 2 electrons and thus form
. The outer most shell of this element has 2 electrons so it loses 2 electrons and thus form 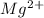 ions. Br is a nonmetal and has 35 atomic number so its electronic configuration is
ions. Br is a nonmetal and has 35 atomic number so its electronic configuration is 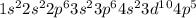 . Since its outermost shell has 7 electrons so it can accept one electron and thus forms
. Since its outermost shell has 7 electrons so it can accept one electron and thus forms 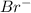 . So magnesium ion and bromide ion combine and forms an ionic compound
. So magnesium ion and bromide ion combine and forms an ionic compound 

