
Chemistry, 19.03.2020 21:44 honeybaby675610
Silver sulfate dissolves in water according to the reaction: Ag2SO4(s) ∆ 2 Ag+(aq) + SO42-(aq) Kc = 1.1 * 10-5 at 298 K A 1.5-L solution contains 6.55 g of dissolved silver sulfate. If addi- tional solid silver sulfate is added to the solution, will it dissolve?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Silver sulfate dissolves in water according to the reaction: Ag2SO4(s) ∆ 2 Ag+(aq) + SO42-(aq) Kc =...
Questions



Computers and Technology, 06.04.2021 16:20

History, 06.04.2021 16:20

Biology, 06.04.2021 16:20

Mathematics, 06.04.2021 16:20

World Languages, 06.04.2021 16:20



Biology, 06.04.2021 16:20

English, 06.04.2021 16:20

English, 06.04.2021 16:20




Mathematics, 06.04.2021 16:20

Mathematics, 06.04.2021 16:20

Mathematics, 06.04.2021 16:20


Mathematics, 06.04.2021 16:20

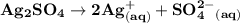
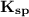 which is =
which is = ![\mathbf{[Ag^+]^2 [SO_4^{2-}]}](/tpl/images/0554/6873/f5bd3.png)
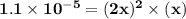
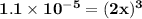
![\mathbf{ x= \sqrt[3]{\dfrac{1.1\times 10^{-5}}{2} } }](/tpl/images/0554/6873/7d563.png)
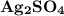 result in the formation of 2 moles of
result in the formation of 2 moles of 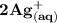
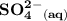
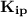 = (0.0280)²(0.0140)
= (0.0280)²(0.0140)

