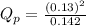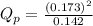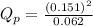Chemistry, 19.03.2020 22:42 Nyasiabaltimore3
He equilibrium constant (KP) is 0.21 at a particular temperature for the reaction:
N2O4(g) <=> 2NO2(g)
Given the following sets of initial conditions, what is the net change that must occur for the reaction to reach equilibrium? Does the reaction shift left to reach equilibrium, does the reaction shift right to reach equilibrium or is the reaction at equilibrium at these initial concentrations so no net change will occur?
Options: Equilibrium, Left, or Right
a. PNO2 = 0.225 atm, PN2O4 = 0.134 atm
b. PNO2 = 0.086 atm, PN2O4 = 0.064 atm
c. PNO2 = 0.130 atm, PN2O4 = 0.142 atm
d. PNO2 = 0.173 atm, PN2O4 = 0.142 atm
e. PNO2 = 0.151 atm, PN2O4 = 0.062 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
He equilibrium constant (KP) is 0.21 at a particular temperature for the reaction:
N2O4...
N2O4...
Questions

Mathematics, 08.05.2021 04:40

History, 08.05.2021 04:40



Arts, 08.05.2021 04:40


Mathematics, 08.05.2021 04:40




Business, 08.05.2021 04:40



Mathematics, 08.05.2021 04:40


Physics, 08.05.2021 04:40





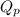 as follows.
as follows.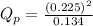
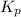 is 0.21 (which is smaller than the value) this means that equilibrium will shift to the left
.
is 0.21 (which is smaller than the value) this means that equilibrium will shift to the left
.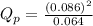
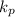 which means that the equilibrium will shift to the right.
which means that the equilibrium will shift to the right.