Chemistry, 19.03.2020 22:32 thomasalmo2014
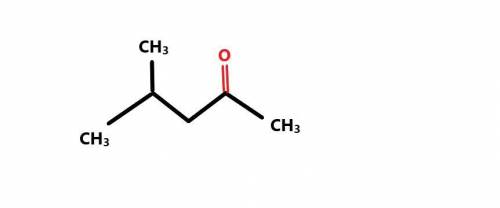
Deduce the structure of an unknown compound using the data below: Molecular Formula: C6H12O IR: 1705 cm-1 1H NMR: no absorptions greater than δ 3 ppm 13C NMR: δ 24.4, δ 26.4, δ 44.2, and δ 212.6 ppm. Resonances at δ 44.2 and 212.6 have very low intensity.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Deduce the structure of an unknown compound using the data below: Molecular Formula: C6H12O IR: 1705...
Questions

History, 06.11.2020 20:30

Social Studies, 06.11.2020 20:30



Mathematics, 06.11.2020 20:30


Mathematics, 06.11.2020 20:30

Mathematics, 06.11.2020 20:30

Mathematics, 06.11.2020 20:30

History, 06.11.2020 20:30

History, 06.11.2020 20:30

Mathematics, 06.11.2020 20:30




English, 06.11.2020 20:30

Social Studies, 06.11.2020 20:30