
Chemistry, 19.03.2020 23:33 littledudefromacross
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simultaneously heated to 35°C. Calculate the entropy change for the process.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simult...
Questions











Mathematics, 17.05.2020 04:57

Mathematics, 17.05.2020 04:57


History, 17.05.2020 04:57






History, 17.05.2020 04:57

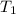 = (19 + 273) K = 292 K,
= (19 + 273) K = 292 K, 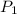 = 1.0 atm,
= 1.0 atm,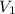 = 1.4 L
= 1.4 L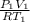
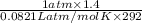
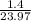
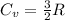
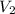 ) of 2.8 L and it is heated to
) of 2.8 L and it is heated to 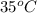 or (35 + 273) K = 308 K.
or (35 + 273) K = 308 K.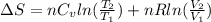
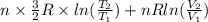
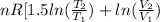
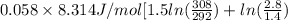
![0.4822 \times [1.5 \times 0.052 + 0.693]](/tpl/images/0554/8697/09f71.png)


