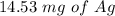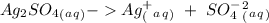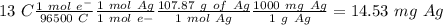Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Suppose a current of 250.mA is passed through an electroplating cell with an aqueous solution of Ag2...
Questions



Health, 25.09.2019 21:30



Mathematics, 25.09.2019 21:30




Mathematics, 25.09.2019 21:30

History, 25.09.2019 21:30


Biology, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30

Chemistry, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30


History, 25.09.2019 21:30

History, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30