
For the reaction CO2(g) + H2O(ℓ) + CaCl2(aq) ←→ CaCO3(s) + HCl(aq) K = 4.8 the concentrations of CO2, H2O, CaCl2 and HCl are 0.377 M, 55.4 M, 0.652 M and 30.2 M, respectively. What will happen in order for the system to reach equilibrium? 1. The reaction will shift to the right. 2. Nothing will occur. 3. The reaction will shift to the left. 4. Not enough information is given.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1
You know the right answer?
For the reaction CO2(g) + H2O(ℓ) + CaCl2(aq) ←→ CaCO3(s) + HCl(aq) K = 4.8 the concentrations of CO2...
Questions

Biology, 13.06.2021 04:40

English, 13.06.2021 04:40


English, 13.06.2021 04:40

Mathematics, 13.06.2021 04:40


English, 13.06.2021 04:40

Biology, 13.06.2021 04:40

Mathematics, 13.06.2021 04:40









Mathematics, 13.06.2021 04:40


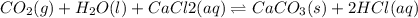
![K=\frac{[HCl]^2}{[CaCl_2]}](/tpl/images/0554/8954/74ccf.png)
![Q=\frac{[HCl]^2}{[CaCl_2]}=\frac{ 30.2^2 }{0.652 }=1398.8](/tpl/images/0554/8954/f4b79.png)


