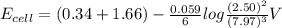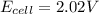Chemistry, 19.03.2020 23:58 naseersaad
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als+3Cus2Al+3aq Suppose the cell is prepared with 7.97 M Cu+2 in one half-cell and 2.50 M Al+3 in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1


Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als...
Questions




Arts, 11.12.2019 23:31

Mathematics, 11.12.2019 23:31


Physics, 11.12.2019 23:31


Mathematics, 11.12.2019 23:31

History, 11.12.2019 23:31




Social Studies, 11.12.2019 23:31






English, 11.12.2019 23:31

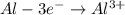 )
)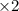 ;
; 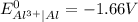
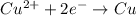 )
) ;
; 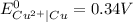
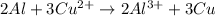
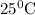 -
-![E_{cell}=[E_{Cu^{2+}\mid Cu}^{0}-E_{Al^{3+}\mid Al}^{0}]-\frac{0.059}{n}log\frac{[Al^{3+}]^{2}}{[Cu^{2+}]^{3}}](/tpl/images/0554/9233/c4d3d.png)