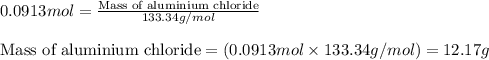Chemistry, 20.03.2020 00:04 janessa0804
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according to the following equation. Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according...
Questions



Chemistry, 20.01.2021 05:40

Mathematics, 20.01.2021 05:40

English, 20.01.2021 05:40

English, 20.01.2021 05:40


Mathematics, 20.01.2021 05:40

English, 20.01.2021 05:40


Geography, 20.01.2021 05:40



Chemistry, 20.01.2021 05:40



Mathematics, 20.01.2021 05:40

Mathematics, 20.01.2021 05:40

Mathematics, 20.01.2021 05:40

Mathematics, 20.01.2021 05:40

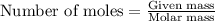 .....(1)
.....(1)
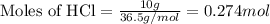
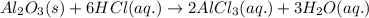
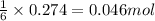 of aluminium oxide
of aluminium oxide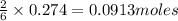 of aluminium chloride
of aluminium chloride