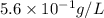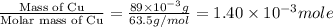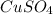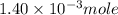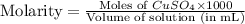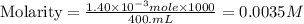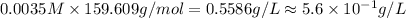Chemistry, 20.03.2020 00:39 xocupcake309174
Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 400.mL copper (II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 89.mg .
Calculate the original concentration of copper (II) sulfate in the sample. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control ch...
Suppose an industrial quality-control ch...
Questions



Mathematics, 04.12.2020 20:00

Social Studies, 04.12.2020 20:00



Social Studies, 04.12.2020 20:00



Mathematics, 04.12.2020 20:00



Mathematics, 04.12.2020 20:00



History, 04.12.2020 20:00

History, 04.12.2020 20:00

Spanish, 04.12.2020 20:00