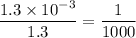Chemistry, 20.03.2020 01:06 blondielocks2002
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and cannot be used in a buffer system. The ratio of acid to conjugate base is outside the buffer range of 10:1. The two species are not a conjugate acid base pair. KF is not soluble in water and cannot be used in a buffer system.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and...
Questions

Mathematics, 21.10.2020 02:01



History, 21.10.2020 02:01



Mathematics, 21.10.2020 02:01



Mathematics, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01

Computers and Technology, 21.10.2020 02:01




Chemistry, 21.10.2020 02:01

Social Studies, 21.10.2020 02:01

Biology, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01

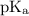 + log
+ log 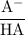
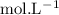 in HF and 1.3
in HF and 1.3 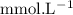 in KF, the ratio is:
in KF, the ratio is: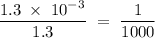
![\text{pH} = \text{pK}_{\text{a}} + \log\dfrac{\text{[A$^{-}$]}}{\text{[HA]}}](/tpl/images/0555/0502/0e377.png)
![\dfrac{1}{10} \leq \dfrac{\text{[A$^{-}]$}}{\text{[HA]}} \leq \dfrac{10}{1}](/tpl/images/0555/0502/63cff.png)